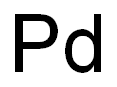Palladium: Applications and preparation
Introduction
Palladium is a chemical element with the symbol Pd and atomic number 46. It is a rare and lustrous silvery-white metal that belongs to the platinum group of elements. Palladium has a number of important uses, including as a catalyst in various chemical reactions, in the manufacturing of electronics, and in jewelry making. One of its most significant uses is in the automotive industry, where it is used in catalytic converters to reduce harmful emissions from gasoline-powered vehicles. Palladium is also used in dentistry, medicine, and groundwater treatment. Due to its rarity and industrial importance, the price of palladium can be quite volatile[1]. Palladium is a silver-white transition metal, soft, has goodductility and plasticity, and can be forged, rolled and drawn. Massive metal palladium can absorb hydrogen gas from stars, causing the volume to expand significantly, become brittle and even break into pieces[1].

Figure 1 appearance of Palladium
Applications
Palladium has a wide range of applications[2-4] due to its unique physical and chemical properties. It is a precious metal that has diverse uses across several industries. One of its primary applications is in automotive catalytic converters, which help reduce harmful emissions from car exhausts and improve air quality. Apart from the automobile industry, It is also used in electronics manufacturing due to its excellent electrical conductivity and resistance to corrosion. It is a common component in electronic devices such as capacitors, resistors, connectors, and switches. In jewelry-making, Palladium is often used as a substitute for platinum since it has similar properties and appearance. It is also a popular material for making dental bridges and crowns in dentistry. Finally, Palladium's unique chemical properties make it useful in various chemical reactions. It is commonly used as a catalyst in organic synthesis and in the production of chemicals and pharmaceuticals.
Preparation
Palladium is a rare and lustrous silvery-white metal that belongs to the platinum group of metals. It is mostly obtained as a by-product of nickel and copper mining. However, it can also be extracted from its ores, such as palladium sulfide (PdS) and palladium chloride (PdCl2). Here are the steps involved in the preparation of Palladium:
Extraction of palladium from its ore:
Palladium can be extracted from its sulfide ore through a process called froth flotation. In this process, the ore is crushed and mixed with water and chemicals such as collectors and frothers. The mixture is then agitated, and air is blown through it to create bubbles. The palladium-containing particles attach to the bubbles and rise to the surface, where they are collected.
Purification of palladium:
After extraction, the crude palladium is purified to remove impurities such as iron, nickel, and copper. This is done through a process called solvent extraction. In this process, the crude palladium is dissolved in a solvent such as hydrochloric acid, and the impurities are selectively removed using an organic extractant.
Reduction of palladium:
The purified palladium is then reduced to its metallic form. This is usually done using hydrogen gas at elevated temperatures. The palladium is heated in a furnace along with hydrogen gas, which reacts with the palladium to form pure metallic palladium.
Refining of palladium:
The final step is refining the palladium to achieve the desired purity level. This is usually done through a process called electrolysis. In this process, the palladium is dissolved in an acid solution and placed in an electrolytic cell. A direct current is passed through the solution, causing the palladium to deposit onto a cathode, while impurities are left behind on an anode. The resulting palladium is highly pure and can be used for a variety of applications, such as catalysis, electronics, and jewelry making[5-9].
References
[1] K. Osseo-Asare, J. R. McCarty, and C. A. Mims III, Handbook of Industrial Crystallization, 2nd Ed., Butterworth-Heinemann, 2002.
[2]Hartwig J F. Palladium-Catalyzed Cross-Coupling Reactions: A General Route to Organic Synthesis[J]. Acc. Chem. Res., 2008, 41(12):1534-1544.
[3]Heck R F. The Role of Palladium in Catalytic Organic Transformations[J]. Angew. Chem. Int. Ed., 2006, 45(48):7896-7903.
[4]Yu J Q. Palladium-Catalyzed C-H Functionalization[J]. Acc. Chem. Res., 2012, 45(8):1227-1236.
[5] Chirik P J, Hartwig J F. Palladium-Catalyzed Suzuki-Miyaura Cross-Coupling Reactions: A Versatile Tool for the Synthesis of Pharmaceuticals, Agrochemicals, and Materials[J]. Science, 2013, 340(6135):613-617.
[6] Rueping M. Palladium-Catalyzed Asymmetric Allylic Substitution Reactions[J]. Acc. Chem. Res., 2010, 43(2):216-226.
[7] J. F. Calvert, The Chemistry of Palladium, Platinum, and Gold, Elsevier, 1997.
[8] H. Nakamura and T. Haishi, "Preparation of Palladium," in Encyclopedia of Inorganic and Bioinorganic Chemistry, Wiley, 2011, 1-15.
[9] R. E. Chinn, "Palladium: Recovery, Properties, and Applications," in Kirk-Othmer Encyclopedia of Chemical Technology, Wiley, 2005, 1-19.
Related articles And Qustion
See also
Lastest Price from Palladium manufacturers

US $2.50-1.00/kg2025-11-03
- CAS:
- 7440-05-3
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 300tons

US $0.00/kg2025-06-05
- CAS:
- 7440-05-3
- Min. Order:
- 1kg
- Purity:
- 99.95%min
- Supply Ability:
- 1 tons